Project
Oligomers as Guest Molecules in Ph-Sensitive Polymer Networks – A Monte Carlo Study
As electrostatic interactions are a driving force for incorporating guest molecules, polyelectrolyte microgels are suitable candidates for drug delivery systems or biosensors. In weak polyelectrolyte microgels, pH controls the degree of ionization within the network and, consequently, the number of incorporated guest molecules. Besides the pH, the degree of ionization depends on many other parameters like the dissociation constants and hydrophobicity of the monomers, microgel, and salt concentration, topology of the network, and also the presence of guest molecules. A systematic investigation of a single parameter can be complicated from the experimental point of view. Instead, varying only one parameter independently in the system can be easily achieved in computer simulations. This fact allowed us to systematically investigate the uptake of weak oligomers in weak polyelectrolyte networks by changing the pH, pK of the network and oligomer monomers, concentrations, chain lengths of oligomers and by adding a salt concentration.
Project Details
Project term
August 1, 2022–December 31, 2023
Affiliations
RWTH Aachen University
Institute
Institute of Physical Chemistry
Project Manager
Principal Investigator
Methods
Since the calculation of electrostatic interactions is computationally time expensive, the usage of High-Performance Clusters was necessary. Furthermore, complexity reduction was achieved by modeling the microgels and oligomers as bead spring polymers. While the solvent was modeled implicitly by a dielectric continuum, the counterions were considered as explicit particles. Molecular Dynamics (MD) simulations are widely used for simulations of microgel systems. However, in classical MD simulations, the number and distribution of charged monomers are fixed. In contrast, not only the positions but also the charge state of the monomers can change within Metropolis Monte Carlo simulations. Here, the degree of ionization directly results from the simulation, and titration curves can be obtained. Besides titration curves, average values of other macroscopic quantities like the radius of gyration of the network or the number of uptaken oligomers can be obtained. Furthermore, microscopic quantities like density functions or the distribution of uptaken oligomers in the network are the results of the simulations.
Results
The simulations showed that the network can uptake oligomers if both are charged. Since both network (acid) and oligomers (base) had weak charges, the uptake was favored at intermediate pH. Moreover, the presence of oligomers enhances both the ionization of the network and the ionization of the oligomers within the network. This effect is stronger in salt-free systems and weaker with adding salt since the charges of the network and the oligomers are screened. The oligomers taken up in the network were homogeneously distributed within the network, and their average degree of ionization was higher than those not taken up. A key result of our simulations was that the number of uptaken charges of the oligomers is a linear function of the network charge if electrostatic interactions are the only driving force for the uptake. The attractive interactions between the oligomers and the network prevented the network from swelling or led even to a slight collapse of the network. The difference in charges of the network and the uptaken oligomers could be interpreted as an effective degree of ionization of the network. This effective degree of ionization increases linearly with the number of counterions within the network and is almost equal for different systems under salt-free conditions.
Discussion
Our simulations demonstrated that the uptake of chains with a higher net charge is favored. Therefore, the network takes up oligomers with more monomer units better than shorter chains. With changing the pK values of the network and the oligomers, the pH range where uptake takes place, and the number of oligomers taken up by the network can be changed. Here, the pK of the network must be smaller or equal to the pK of the oligomers. In the other case, no uptake takes place. If the pK of the network is much smaller than the pK of the oligomers, more chains are taken up, and uptake was found over a broader pH range. Adding salt led to the screening effect. As a consequence, fewer oligomers were taken up, and the pH range of uptaken oligomers shrunk.
Increasing the microgel and the oligomer concentration facilitated the uptake of the oligomers at lower pH values and increased the number of uptaken oligomers at the isoelectric point. At high pH, fewer chains were uptaken since the charge compensation of the network charges by the counterions instead of the oligomers was more favored at higher concentrations. The compensation by the counterions is enhanced since the entropic gain of being outside the network is smaller for higher microgel concentrations. Adding salt weakened the influence of microgel and oligomer concentration.
Additional Project Information
DFG classification: 306-02 Natural Life Sciences, Polymer Research, Experimental and Theoretical Physics of Polymers
Cluster: CLAIX
Publications
Strauch C, Schneider S, Oligomers as guest molecules in pH-sensitive polymer networks – a Monte Carlo study, manuscript in progress.
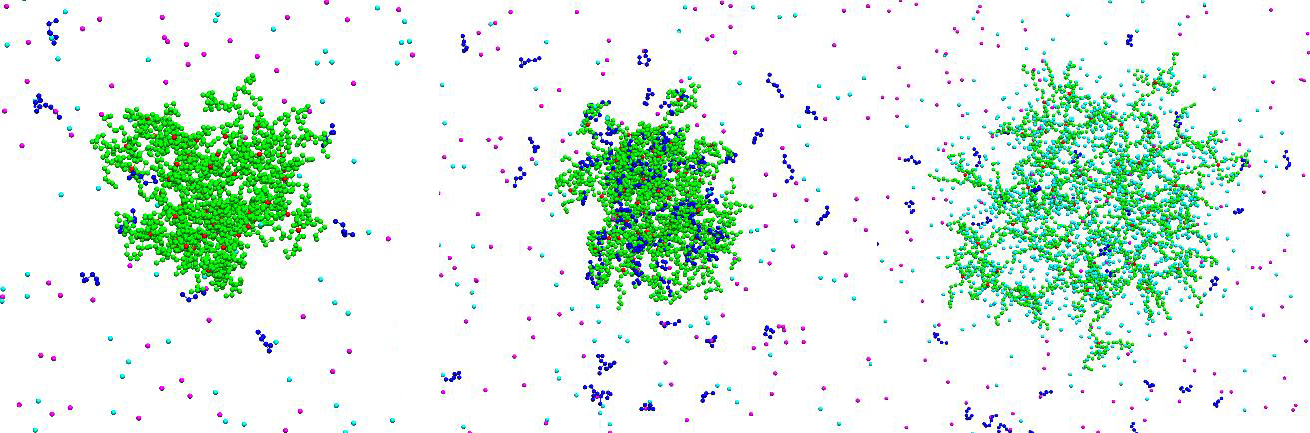 Figure 1: Snapshots of uncharged network at pH 4(left), charged network and uptaken chains at pH 7 (middle) and swollen network at pH 11 (right).
Figure 1: Snapshots of uncharged network at pH 4(left), charged network and uptaken chains at pH 7 (middle) and swollen network at pH 11 (right).