Project
Mechanistic Investigations on the Hydrogenative Reduction of Carbon Dioxide
Computer simulations, specifically Density Functional Theory (DFT) methods, play a crucial role in catalyst design. Catalysts are essential in accelerating chemical reactions and finding efficient, environmentally friendly processes. Traditional experimental methods for catalyst design can be time-consuming, costly, and limited by practical constraints. However, DFT-based computer simulations provide invaluable insights into the atomic-level interactions, reaction mechanisms, and energetics involved in catalysis. By harnessing the power of computational modeling, scientists can expedite the discovery and optimization of catalysts, leading to innovative and sustainable solutions for various industries, such as energy, pharmaceuticals, and environmental applications. In this context, the importance of computer simulations like DFT methods cannot be overstated, as they have revolutionized catalyst design and have the potential to drive advancements that positively impact our society and the environment. To find a transition metal catalyst for the reverse water gas shift reaction (rWGSR), the significance of computer simulations, particularly DFT methods, becomes even more pronounced. The reverse water gas shift reaction involves the conversion of carbon dioxide (CO₂) and hydrogen (H₂) into carbon monoxide (CO) and water (H₂O). This reaction is of importance in the field of sustainable energy and carbon capture utilization, as it can serve as a vital step in the production of valuable fuels and chemicals while mitigating greenhouse gas emissions. Experimentally identifying an efficient and selective transition metal catalyst for the rWGSR can be a challenging and time-consuming task. By employing DFT methods, researchers can model the electronic and atomic structures of various transition metal catalysts and their interactions with reactants and intermediates during the rWGSR. These simulations can predict crucial thermodynamic and kinetic parameters, such as reaction barriers, activation energies, and rate constants, which are difficult to obtain through traditional experimental observations. Furthermore, DFT simulations enable researchers to explore a vast chemical space and screen numerous transition metal catalyst candidates rapidly. The ability to gain valuable insights into the reaction mechanisms and understand the factors influencing catalytic activity and selectivity is invaluable for catalyst design and optimization. In addition to exploring known transition metals, DFT simulations can suggest promising novel catalytic materials and guide experimental efforts in their synthesis and characterization. Overall, the integration of computer simulations, especially DFT methods, in the search for a transition metal catalyst for the rWGSR reaction offers a powerful and efficient approach to accelerate the discovery of highly efficient and selective catalysts. As we move towards a more sustainable future, finding effective catalysts through computational modeling will play a crucial role in advancing clean energy technologies and addressing environmental challenges.
Project Details
Project term
April 12, 2022–May 5, 2023
Affiliations
RWTH Aachen University
Institute
Translational Molecular Catalysis
Project Manager
Principal Investigator
Methods
Herein, we use DFT to study our novel catalysts to understand limitations and opportunities in further catalyst design for such reactions. Structure optimizations of the reactants, intermediates, products, and transition state (TS) structures were carried out with the Turbomole 7.0.1 program on the high-performance compute cluster of the RWTH-Aachen University. These geometry optimizations were performed using the tpss functional and def2-SVP basis set and a grid size of m4, as implemented in the Turbomole program package. Intrinsic reaction coordinate (IRC) calculations were performed on the TS structures in order to connect the localized minima to the respective TS. For a more accurate description of the potential energy surface, consecutive single point calculations, employing the M06-L functional and the def2-TZVPP basis set with implemented ECP for Pd in conjunction with conductor-like polarizable continuum model (CPCM) and methanol solvent, were performed using the Gaussian 16 (revision b.01) program. The browser based front-end Jupyter Notebook was used to handle the data for the evaluation of the catalyst’s performance and construction of molecular volcano plots. Linear free energy scaling relationships were established by calculating the thermodynamic stabilities for each intermediate and transition state relative to a reference state structure.
Results
With the focus on establishing a consistent reaction mechanism, we were able to identify the rate-determining states during catalysis by finding the highest energy span in the cycle. We concluded a plausible reaction pathway for the reverse water gas shift reaction which was complying to our experimental data. Furthermore, specific experimental observations could be explained with the determined mechanism. A crucial structural conformation of the catalyst could be identified as lead structure enabling the CO₂ activation. We investigated several metal-ligand combinations, resembling the required structural conformation, from which we identified four promising catalyst structures that were able to reduce CO₂ by rWGSR.
Discussion
Due to computational methods we discovered a new mechanistic pathway enabled by the structural characteristics of certain ligands. A palladium catalyst showed the possibility of a hydrogen transfer initiated by the ligand’s secondary amine functionality. To determine the influence of the phosphine substituents on the decarbonylation step of the investigated reaction mechanism, additional DFT calculations were performed with a variation of electron withdrawing and electron donating substituents. The calculations are consistent with the tendencies which were observed in the experiments. Electron donating substituents increase the electron density at the palladium center, leading therefore to a stronger π backdonation to the CO ligand and may hinder its liberation. Based In these findings two further palladium catalysts and a nickel catalyst could be developed.
Additional Project Information
DFG classification: 403-02 Engineering Sciences Technical Chemistry
Software: Gaussian16, TURBOMOLE, Jupyter Notebook
Cluster: CLAIX
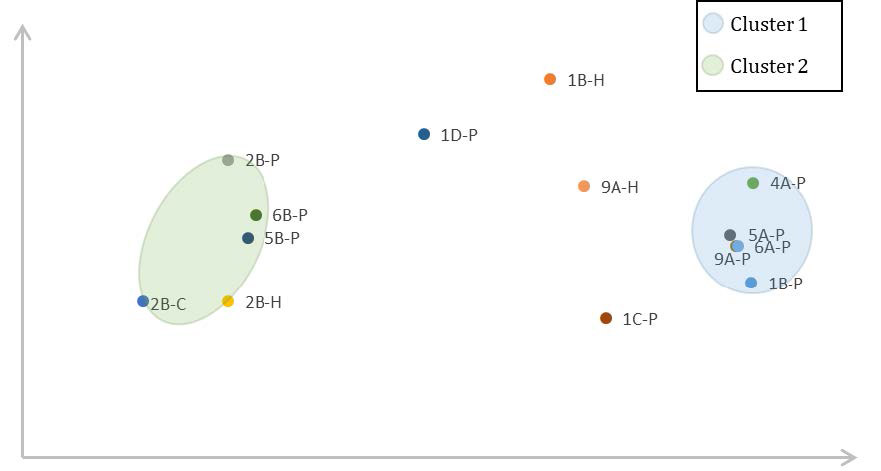 Figure 1: Relative energy barrier ∆GTS2 in relation to the metal-CO₂ bond distance for chosen catalyst complexes. P = proton pathway,
H = hydride pathway, C = concerted pathway
Figure 1: Relative energy barrier ∆GTS2 in relation to the metal-CO₂ bond distance for chosen catalyst complexes. P = proton pathway,
H = hydride pathway, C = concerted pathway