Project
Ligand selectivity and activation mechanism of the oxytocin receptor and impact of a disease-linked mutation
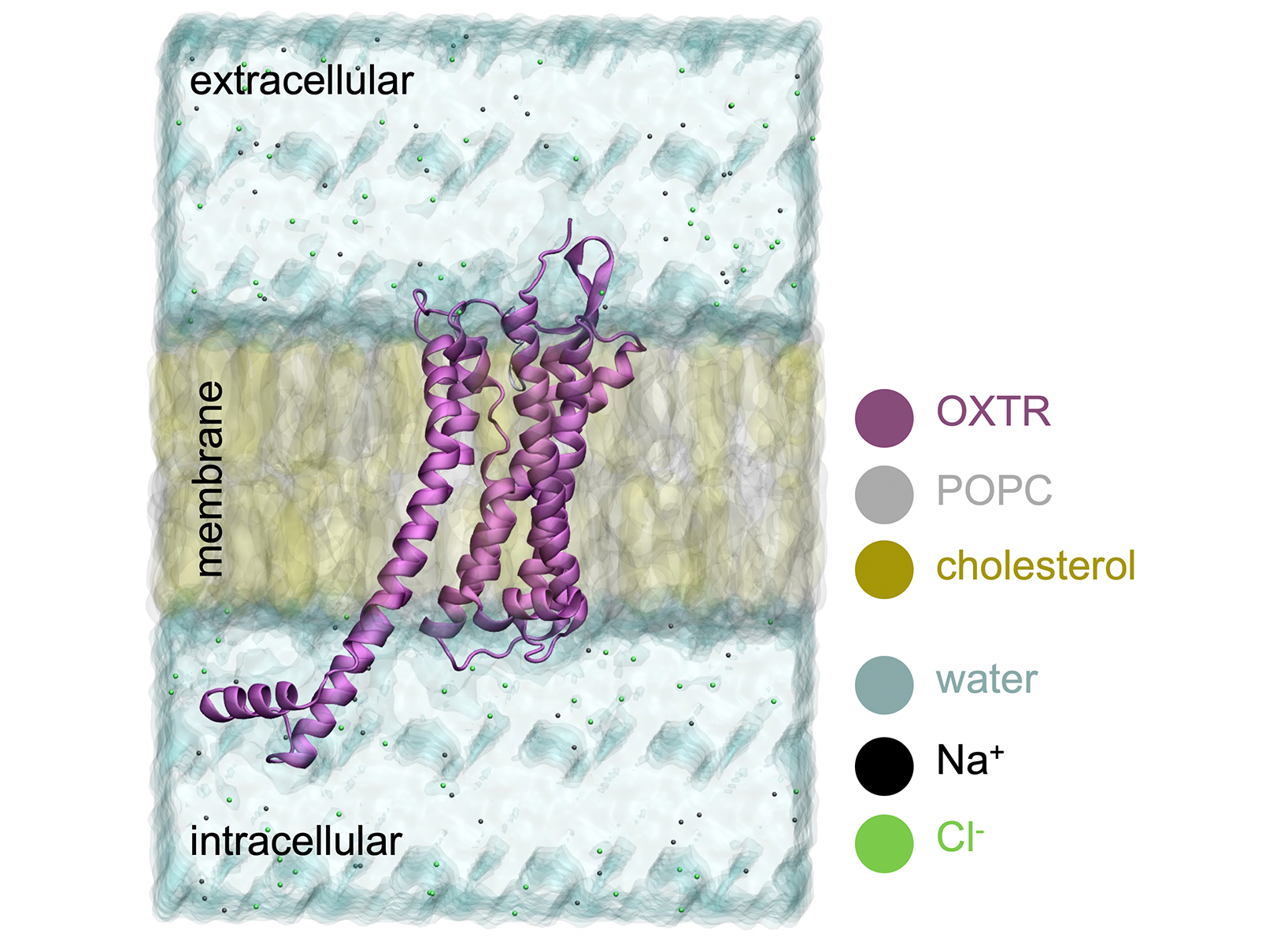
G protein-coupled receptors (GPCRs) are the largest family of cell membrane receptors and are targeted by over 30% of currently available drugs. Among them, the oxytocin receptor (OXTR) is activated by the neuropeptide oxytocin (OXT), the so-called love hormone, and thus is involved in social behavior, among other functions. Mutations on the OXTR, in particular the single nucleotide polymorphism Ala218Thr, have been associated to autism spectrum disorder. In 2022, a combined modeling-experimental study by some of us suggested that the Ala218Thr mutation could affect receptor activation; however, an in-depth study of the dynamics of the receptor is crucial to confirm and fully understand such effect. Moreover, obtaining good statistics from the molecular dynamics trajectories requires several microsecond long replica simulations, for both the wild-type and mutant variants of the receptor. Hence, such simulations can only be performed in HPC resources, such as CLAIX-2018.
Project Details
Project term
June 26, 2023–December 6, 2023
Affiliations
Marburg University
Institute
Analytical Chemistry, Marburg University
Methods
In particular, in this project we combined molecular dynamics (MD) simulations with the Replica Exchange with Solute Tempering (REST2) method, a Hamiltonian replica exchange scheme that enhances MD sampling without the need of defining any collective variable or reaction coordinate.
Results
We first investigated the effect of the Ala218Thr mutation on the basal activity of the receptor by running REST2 simulations on apo OXTR (i.e. the form devoid of ligand and thus in the inactive state), for both wild-type and mutant receptor. Analysis of the backbone RMSD with respect to the initial structure shows that the wild-type receptor explores only one main (inactive) conformation, whereas the mutant receptor shows two populations, one similar to the wild-type and another at lower RMSD values, indicating that the Ala218Thr mutation modifies the conformational space of the apo receptor. However, we did not observe any transition towards the active state, which might be due to the absence of the endogenous agonist. Therefore, we next studied the effect of the Ala218Thr mutation on the OXTR-OXT complex. To assess receptor activation, we monitored during the simulations the distance between transmembrane helices 2 and 6 (TM2-TM6) on the intracellular part of the receptor. An increase in the TM2-TM6 distance was observed when wild type OXTR was embedded in a pure phospholipid membrane, indicating that the intracellular part of the receptor opens, but does not reach a fully active state. As cholesterol has been reported to be an allosteric modulator of OXTR activation, we also performed REST2 simulations with the receptor embedded in a mixed phospholipid:cholesterol membrane. The TM2-TM6 distance did not significantly change, indicating that wildtype OXTR remains in the inactive state. In contrast, for the Ala218Thr mutant OXTR-OXT complex, two states were observed for both membranes with and without cholesterol, corresponding to inactive and intermediate (on-its-way-to-active) states. This indicates that the mutation also modifies the conformational landscape of the receptor-peptide complex, even though our REST2 simulations failed to sample the fully active state. Aiming at improving the sampling of the active state, we decided to adjust our model to take into account the positive allosteric effect of magnesium on receptor activation. Unbiased MD simulations were run for the active state of the wild-type receptor in complex with oxytocin and Mg2+, embedded in either a pure phospholipid or a mixed phospholipid:cholesterol membrane. These simulations showed that the most flexible region of the receptor corresponds to TM5 and TM6, as well as the intracellular loop (ICL3) connecting them. In addition, the Mg2+ ion is stable inside the binding site, coordinated by both receptor and peptide residues, as well as water molecules. Finally, cholesterol occupies different regions around the receptor, in line with the lipid densities observed experimentally in the cryo-EM structures.
Discussion
Based on the aforementioned simulation results, we conclude that OXTR activation depends on the interplay of
different factors, including the presence of the endogenous OXT agonist and allosteric modulators such as
cholesterol and magnesium. Further enhanced sampling simulations will be needed to fully explore the
conformational transition between active and inactive states of the receptor, possibly including the effector G
protein.
Additional Project Information
DFG classification: 201 Basic Research in Biology and Medicine, 206 Neurosciences
Software: Gromacs, PLUMED
Cluster: CLAIX
Publications
Kanso A, Ligand binding and activation mechanism of 7-transmembrane receptors, Master thesis, University
of Montpellier, September 2023.
Al Sadi S, Molecular understanding of magnesium ion-mediated ligand binding to the oxytocin receptor using
molecular dynamics simulations, Master thesis, University of Bologna, March 2024.