Project
Modeling nanometer-sized pores for protein detection
Nanopores are nanometer-sized apertures that function as single-molecule biosensors and have achieved significant success in label-free single-molecule DNA, RNA, protein and glycans sequencing. In a typical nanopore sequencing setup, a transmembrane bias potential is applied across the nanopore, allowing for the measurement of longitudinal ionic current traces. As individual analytes pass through the nanopore under the applied potential, they block the flow of ions, which is reflected in the time-dependent current traces through the pore. By analyzing the amplitude and duration of these blockade currents, this technique enables the precise identification of analytes at the single-molecule level, providing a cost- and time-efficient, as well as a label-free method.
Project Details
Project term
August 1, 2024–January 31, 2026
Affiliations
RWTH Aachen University
Institute
Computational Biotechnology
Principal Investigator
Methods
All-atom molecular dynamics (MD) simulations were performed using GROMACS to model graphite-based biomimetic nanopores immersed in 1 M KCl solution. An external electric field applied along the z-axis facilitated analyte translocation. A model glycan with the sequence was built and threaded through the nanopore. The Amber force field described the protein, while nanopore parameters followed previous setups. The system, containing ~200,000 atoms, was equilibrated under NVT and NPT ensembles with harmonic restraints CA atoms. A 1.5 micro-sec production simulation under a –1 V transmembrane voltage was conducted, followed by an independent replicate with random velocities, yielding consistent results.
Results
Main objectives included in this proposal with delay. After overcoming these challenges, we made substantial progress on the detection of proteins and glycans using the nanopore platform and are now preparing manuscripts for submission. Here we briefly discuss the results of glycan sensing. As fundamental biomolecules, glycans derive their physiological and pathological functions from their intricate structural diversity, which poses significant challenges for sequencing. Moreover, glycan sequencing using nanopore technology remains largely unexplored. To address this, we have developed a novel biomimetic nanopore made of graphite, designed to mimic biological protein nanopores. Understanding the atomistic and dynamic interactions of glycans within the graphite-based nanopore is crucial for efficiently translocating and detecting them. For this purpose, we will employ all-atom MD simulations in the presence of an electric field. Indeed, we successfully identified a variety of complex glycans exhibiting different numbers of functional groups, chain lengths ranging from tetrasaccharides to hexasaccharides, and distinct glycosidic linkages (Figure 1). Our simulations revealed distinct charge density signatures for glycans containing beta-1,3 or beta-1,4 glycosidic bonds within the nanopore, enabling the discrimination of individual glycan isomers. At the same time, the field has evolved providing with new insights and ideas, so that it was natural extend the used of the graphite nanopore for sensing into using this for neuromorphic memristor devices. In short, we developed a bipolar iontronic memristor composed of graphite–hydrogel–graphite layers functioning within an electrolyte environment, where the central hydrogel layer is composed of electrically neutral, soft, and biocompatible poly (lactic-co-glycolic acid) (PLGA) polymer (Figure 2). Our all-atom MD simulations reveal that the proposed device exhibits distinctive memristive behavior, including a hysteretic current–voltage response under applied transmembrane voltages. Notably, the central dense hydrogel layer plays a pivotal role in inducing in-channel ion concentration polarization and also influences on ion transport by selectively trapping cations through electrostatic interactions with its negatively charged oxygen atoms. In addition, we systematically investigate the effects of electrolytes, nanopore surface charge, and hydrogel porosity on device performance. Stronger hysteresis in ionic current is observed with KCl compared to NaCl, while excessive nanopore charge reduces hysteresis due to ion saturation in the central hydrogel layer. Decreasing hydrogel porosity alters the ion mobility; preserves though the memristive behavior. Altogether, these findings offer molecular-level insights and design strategies for realizing and optimizing soft-matter-based iontronic memristors for relevant neuromorphic applications. The preliminary results from this study are highly promising, and the corresponding manuscript is currently under peer review. We anticipate receiving reviewer comments soon and will require these computational resources to perform additional simulations probably needed for the revision process.
Discussion
The computational modeling associated with these ongoing studies remains highly relevant to this computing project. We are currently finalizing several additional simulation results and planning to perform free energy calculations to gain a deeper understanding of the translocation process through the nanopore. These simulations will help characterize the energetic landscape and reveal key molecular interactions that govern analyte transport at the atomic level. The insights obtained will complement our existing findings on nanopore-based detection mechanisms and contribute to refining the design of biomimetic nanopore systems. Overall, this work will significantly advance the computational and mechanistic objectives outlined in the current project. Therefore, we would like to request an extension of the p0022407 account, if possible, for a minimum of 6 months to a maximum of 12 months.
Additional Project Information
DFG classification: 201-02 Biophysics
Software: Gromacs
Cluster: CLAIX
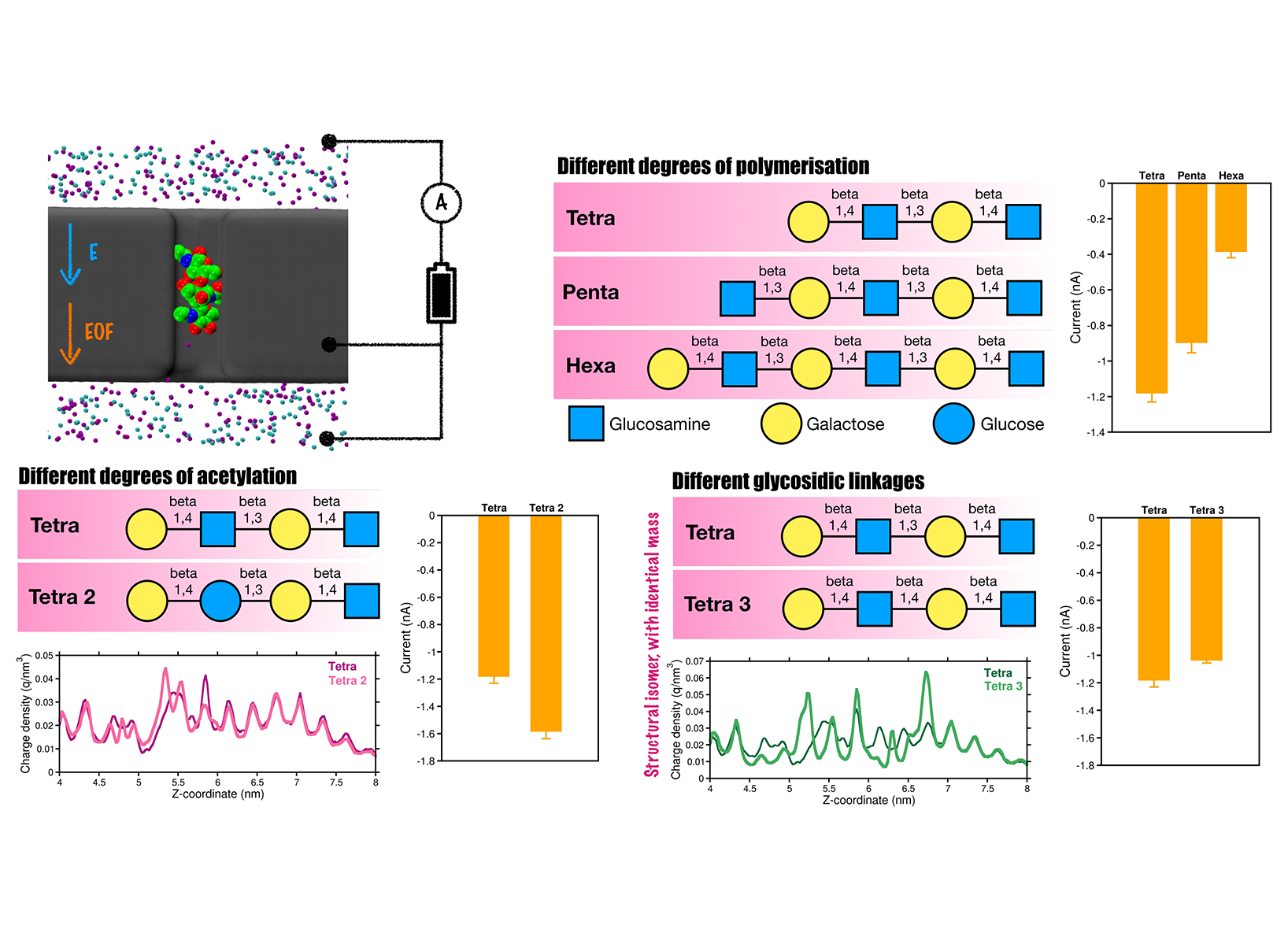 Our modeled nanopore system for glycan sensing. Distinct current profiles are observed for complex glycans differing in degree of polymeriza- tion, glycosidic linkages, and isomeric forms.
Our modeled nanopore system for glycan sensing. Distinct current profiles are observed for complex glycans differing in degree of polymeriza- tion, glycosidic linkages, and isomeric forms. 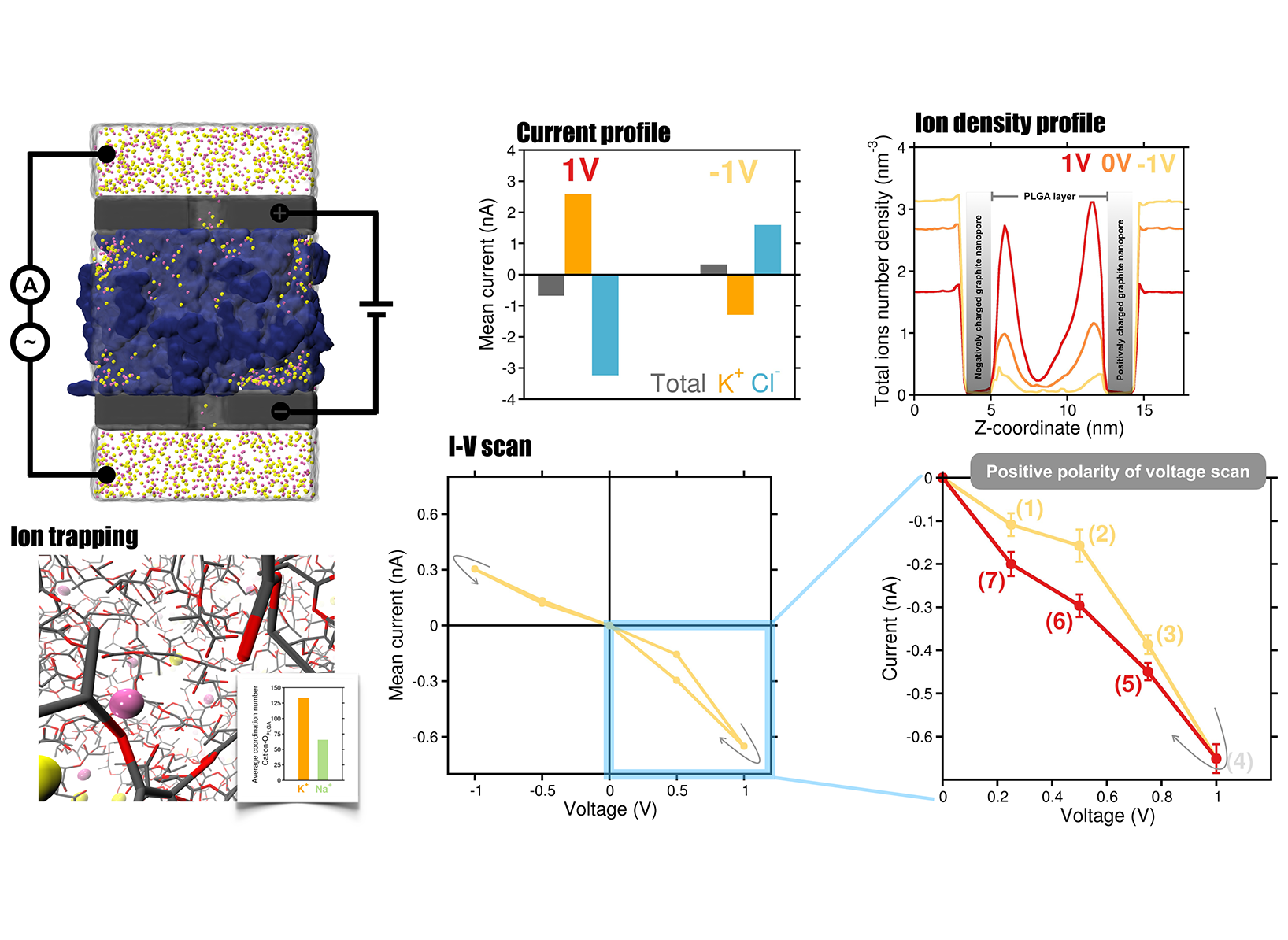 Our modeled graphite–hydrogel–graphite memristor system. The figure shows the current profiles, ion density distribution, and distinct hys- teretic current-voltage characteristics of the memristor.
Our modeled graphite–hydrogel–graphite memristor system. The figure shows the current profiles, ion density distribution, and distinct hys- teretic current-voltage characteristics of the memristor.