Project
Extending quantum-chemical maps to complex solids
Predicting the properties of solids is an important goal to tailor advanced functional materials such as thermoelectrics, phase change materials, topological insulators, and others. A concept based on the close link between chemical bonding and material properties has offered the successful prediction of various properties as well as a classification of the types of bonding. To utilize this relationship, it is necessary to precisely quantify chemical bonding in solids. We have therefore compared quantum-chemical bonding descriptors determined by several recently developed techniques, i.e., the transfer of electrons and the formation of electron pairs between adjacent atoms as shown in Figure 1a. This map has proven effective in distinguishing/predicting unique materials based on their bond rupture behavior as shown in Figure 1b. Toward a universal map, more complex solids should be included into the map, which is the goal of the present proposal.
Project Details
Project term
December 5, 2023–December 4, 2024
Affiliations
RWTH Aachen University
Institute
Institute of Physics
Project Manager
Principal Investigator
Methods
In this project, physical and chemical properties of solids are characterized with Density Functional Theory (DFT). Density Functional Theory is a quantum mechanical method to investigate the electronic structure of many-body systems (principally the ground state properties). The modern formulation of DFT originated from the famous paper written by Hohenberg and Kohn in 1964, where they showed the ground state electron density can be used as a basic variable, and all the ground state properties of the system can be considered to be unique functionals of such variable. Later in 1965, another classic work by Kohn and Sham has converted the original interacting many-body problem to an auxiliary non-interacting particle problem, which established the framework for present-day electronic structure calculations.
Results
Successful predictions of various properties via bond descriptors in s- and p-electron materials have demanded an extension of material classes utilizing d-electrons, including transition metal dichalcogenides (TMDCs). However, the large complexity due to the increased number of interactions between s-, p-, and d-electrons complicates the interpretation on the descriptors. Recently, we successfully clarified descriptors with orbital resolved manner as shown in Figure 2. Compared to the total ES values (Figure 2a), those corresponding to the p-d interactions (Figure 2b) exhibit distinct differences. Properties follow the trends of ES values such as stacking fault energy, the degree of suppression in the vdW gap, and bond angle distributions. Presently, a publication is in preparation where this idea is extended further to classify solids. Within the scope of this project, we would like to extend the framework discussed before to materials whose bonding mechanism involves the participation of d-electrons. Bond descriptors of compounds which involve d-orbitals as a major contribution to their bond show a different distribution pattern since often more than one type of orbital per atom is relevant for bond formation. Thus, to ensure that the concepts developed can also be applied in this case, we would also like to investigate the behavior of d-electrons in bonds. Combining property-based and descriptor-based classifications, we hope to provide a comprehensive understanding on the types of bonding in d-orbital compounds. Understanding the bonds involving d-electrons seems to be the key to unravel a range of phenomena, such as soft bonds in superionic
conductors, important for novel battery concepts, and novel design approaches to increase the refractive index.
Additional Project Information
DFG classification: 303 Physical and Theoretical Chemistry, 307 Condensed Matter Physics
Software: QuantumEspresso, VASP, Critic2, DGRID, LOBSTER
Cluster: CLAIX
Publications
Ruixuan Chu et. al., Ab initio investigation of amorphous and crystalline arsenic sesqui-chalcogenides: optical properties explained by metavalent bonding, PSS-RRL 2400311 (2024)
Felix Hoff et. al., Bond confinement dependent Peierls distortion in epitaxially grown Bismuth films, Advanced Materials, 2416938 (2024)
Oana Cojocaru-Mirédin et. al., Atom Probe Tomography: a local probe for chemical bonds in solids, Advanced Materials, 2403046 (2024)
Guodong Tang et. al., Interplay between metavalent bonds and dopant orbitals enables the design of SnTe thermoelectrics,
Nature Communications, 15, 9133 (2024)
Changwoo Lee et. al., Ultrahigh Stability and Operation Performance in Bi-doped GeTe/Sb2Te3 Superlattices Achieved by Tailoring Bonding and Structural Properties, ACS Nano, 18, 25625-25635 (2024)
Jean-Yves Raty et. al., Changes of bonding upon crystallization in phase change materials, PNAS, 121 (19) e2405294121 (2024)
Peter Kerres et. al., Growth of textured chalcogenide thin films and their functionalization through confinement, Physica Status Solidi A 2300921 (2024)
Nan Lin et. al., Metavalent Bonding in Cubic SnSe Alloys Improves Thermoelectric Properties over a Broad Temperature Range, Adv. Funct. Mater., 34, 2315652 (2024)
Decheng An et. al., Metavalently bonded tellurides: the essence of improved thermoelectric performance in elemental Te, Nature Communications 15:3177 (2024)
Jean-Yves Raty et. al., Tailoring chemical bonds to design unconventional glasses, PNAS, 121 (2) e2316498121 (2024)
Matthias Wuttig et. al., Metavalent or Hypervalent Bonding: Is There a Chance for Reconciliation? Adv. Sci., 11, 2308578 (2024)
Thesis
Tim Bartsch Master, Atoms in chains: Pressure-Induced Structural Transitions and Their Impact on Properties in 1D and 3D Materials, Master’s thesis on-going.
Johannes Holters, Charge Density Wave Dynamics and Topological Properties in CuTe Thin Films, Master Thesis
Xin Zhong, Metavalent Bonding: A Universal Strategy to Enhance the Thermoelectric Performance of GeSe compounds, Master Thesis
Zhenying Su, Trends of thermal conduction depending on the types of bonding: metavalent, covalent, metallic and ionic bonding, Master Thesis
Timo Veslin, Investigation of ultrafast dynamics in metavalently bonded Bi thin films, Master Thesis
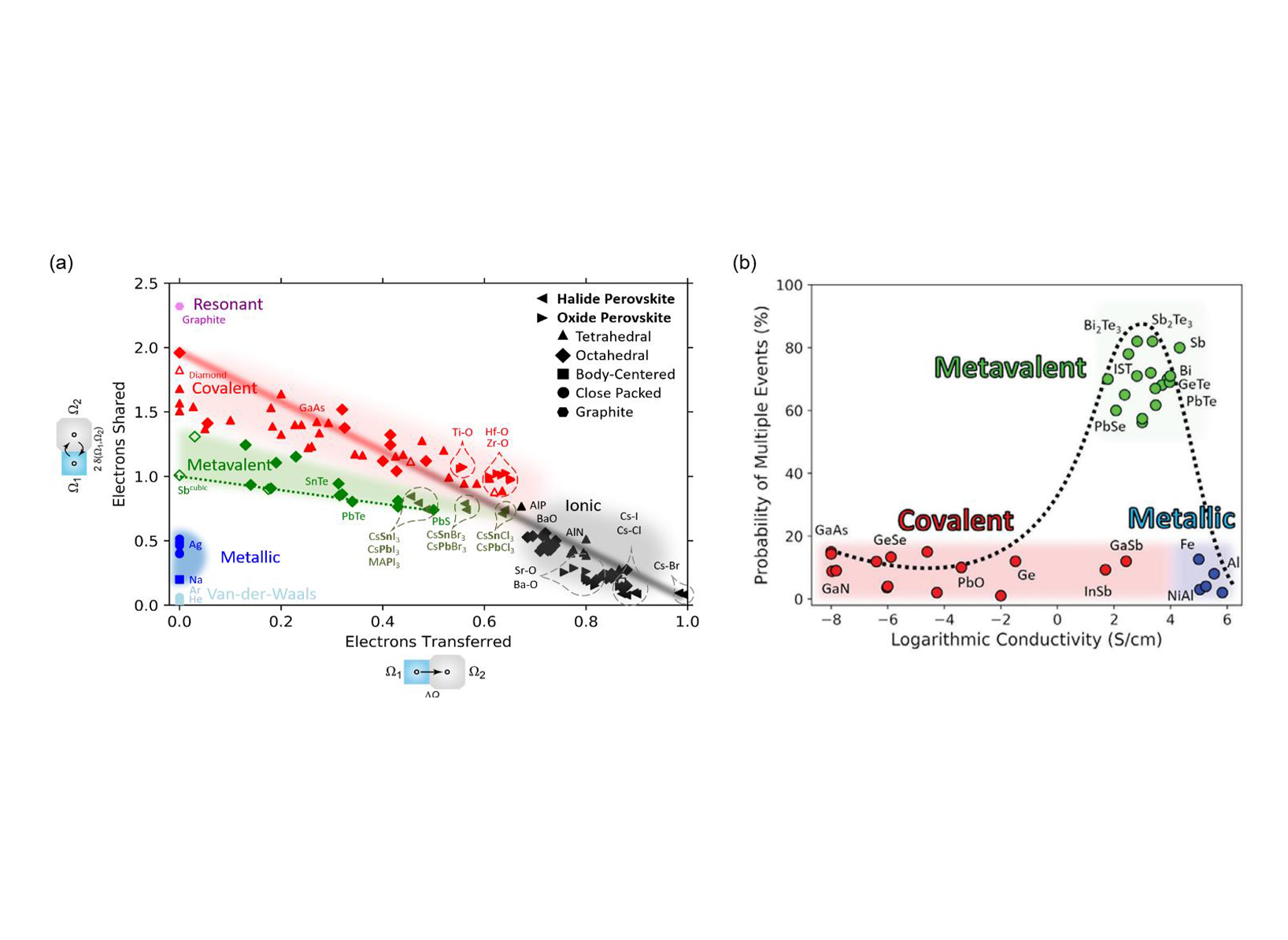 Figure 1(a) 2D map describing bonding in solids. (b) Bond rupture behavior. The map is spanned by the renormalized electron transfer between adjacent atoms, obtained after division by the formal oxidation state and the sharing of electrons between them. Triangles, diamonds, squares and circles denote tetrahedrally bonded solids, distorted and ideal rocksalt-type (octahedrally coordinated) structures, body-centered solids and close-packed metals, respectively, while filled and open symbols represent thermodynamically stable and metastable phases. Triangles pointing to the left denote Halide Perovskites, triangles pointing to the right oxide perovskites. All ideal rocksalt structures for materials with half-filled p-bands are located on one line, spanning from AgSbTe2 to PbSe, while all distorted octahedrally coordinated structures are situated above it, characterized by a larger number of electrons shared. Half-filled p-band crystals demonstrate distinct behavior in bond rupture. (Adv. Funct. Mater. 2022, 32, 2110166; Adv. Mater. 2024, 2403046)
Figure 1(a) 2D map describing bonding in solids. (b) Bond rupture behavior. The map is spanned by the renormalized electron transfer between adjacent atoms, obtained after division by the formal oxidation state and the sharing of electrons between them. Triangles, diamonds, squares and circles denote tetrahedrally bonded solids, distorted and ideal rocksalt-type (octahedrally coordinated) structures, body-centered solids and close-packed metals, respectively, while filled and open symbols represent thermodynamically stable and metastable phases. Triangles pointing to the left denote Halide Perovskites, triangles pointing to the right oxide perovskites. All ideal rocksalt structures for materials with half-filled p-bands are located on one line, spanning from AgSbTe2 to PbSe, while all distorted octahedrally coordinated structures are situated above it, characterized by a larger number of electrons shared. Half-filled p-band crystals demonstrate distinct behavior in bond rupture. (Adv. Funct. Mater. 2022, 32, 2110166; Adv. Mater. 2024, 2403046) 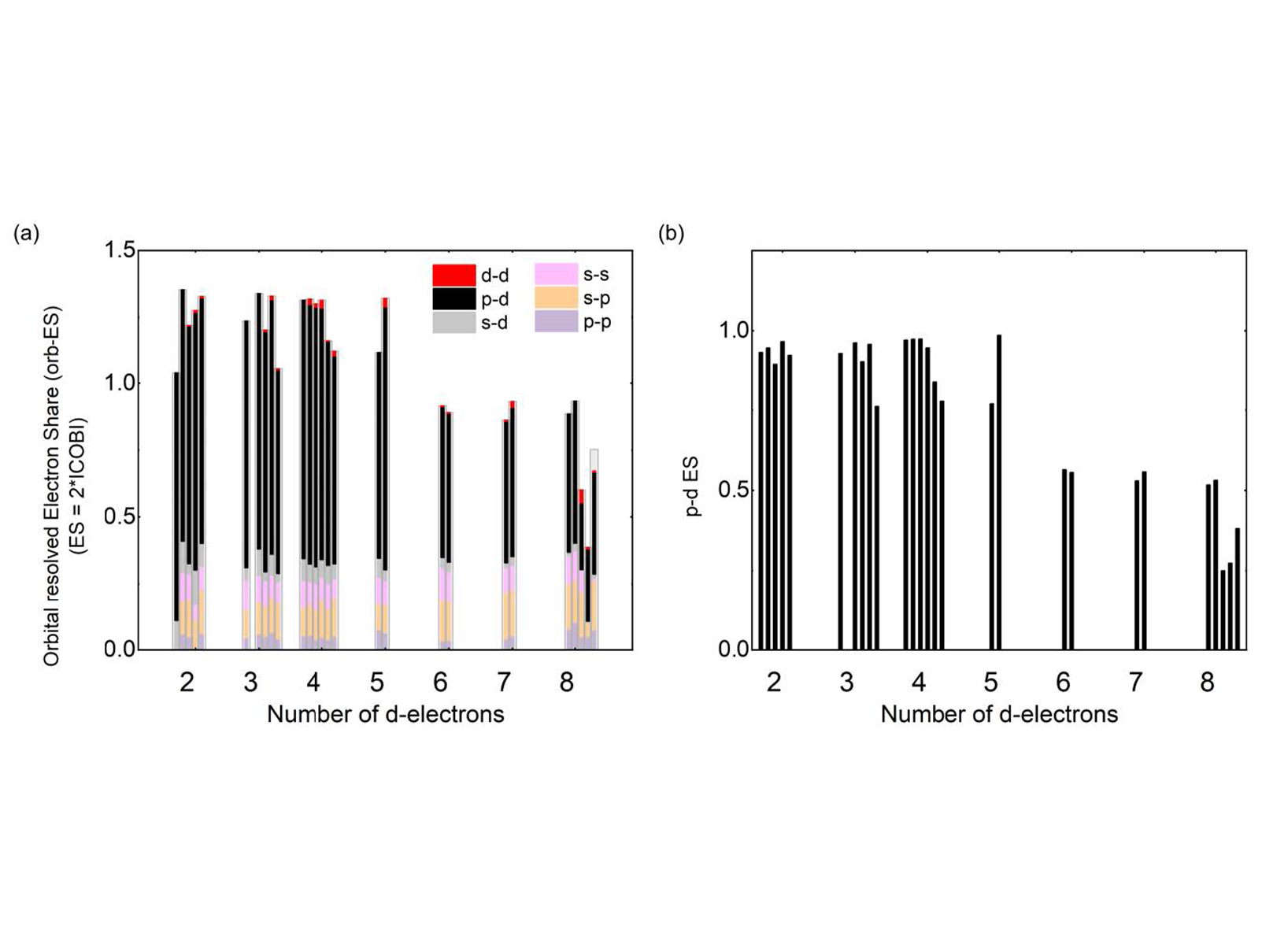 Figure 2. Orbital resolved electrons shared (ES) for TMDCs. (a) Total contribution to ES and the orbital-resolved contributions to this quantity. (b) Contribution to ES from the electron overlap between p- and d-orbital electrons.
Figure 2. Orbital resolved electrons shared (ES) for TMDCs. (a) Total contribution to ES and the orbital-resolved contributions to this quantity. (b) Contribution to ES from the electron overlap between p- and d-orbital electrons.